In this Circuit Showcase, we’re starting at the absolute beginning. If you’re looking to bootstrap your understanding of electronics and the physical phenomena of electricity, you’re in the right place!
Disgruntled chemists, and the art of mooching
Two chemists walk into a bar. The first says to the bartender, “I’ll have a glass of H2O.” The second chemist dies.
Now that we’ve cleared out all the chemists, let’s address the elephant in the room. You’ve gotta know just a little bit of basic chemistry to begin grappling with the idea of electricity. Thankfully, we don’t need to know much. Engineers, long ago, lead chemists into dark alleys to thump them over the head and root around in their pockets for formulae. We can steal and enjoy the punchlines, and not worry about the rigorous science behind them.
If you grew up with Bill Nye, you already know that everything is made of stuff called matter, and that matter is made up of atoms of various elements. Atoms, in turn, are made up of a compact nucleus, and surrounded by a cloud of particles called electrons. We’re already halfway there, really.
Behold: a model of the silicon atom. This Bohr model of the atom is sufficient for us, but it needs to be said that this is only a model, a representation, and doesn’t reflect reality all too accurately. Thankfully, it is a distinction we are only too happy to leave to the egg-heads in lab coats.
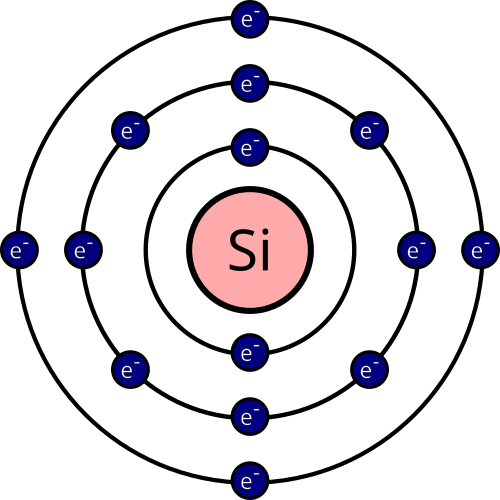
The nucleus is made up of uncharged, neutral neutrons and positively charged protons. Neutrons and protons are tightly bound in the nucleus and cannot move or leave the atom. They’re so unimportant to us, we don’t even bother to draw them as individual particles in the figure above; they simply make up the net positively charged nucleus in the centre. Surrounding the nucleus is a cloud of negatively charged particles called electrons. Electrons are organised into shells around the nucleus, and for the most part, only the electrons of the outer-most valence shell are free to engage in reactions. Unlike the neutron and the proton, valence electrons are free to move around and, in some cases, leave their atom. Chemistry, unless you’re splitting the atom, is all about the interactions of these electrons between atoms.
And that’s just about all the chemistry you need to know!